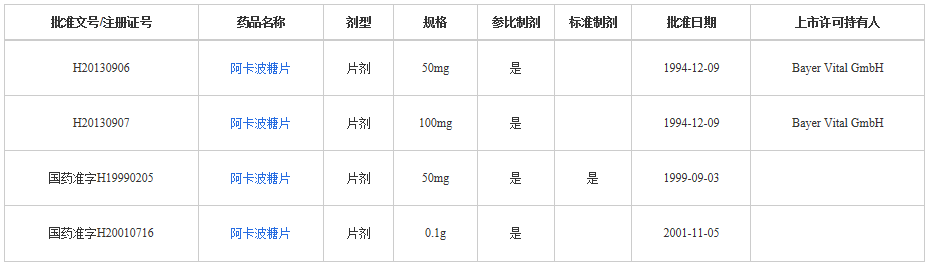
At present, all listed in the list of listed drugs are produced by Bayer!
Related reading:
Bayer Group, China's regional prescription drug business sales in 2017 about 17 billion yuan, an increase of 18.3% over the previous year.
The performance of Bayer's prescription drug business in China in 2016 is 1.8 billion euros (more than 13 billion yuan in total). In 2015, 1.6 billion euros, about 11.4 billion yuan in sales.
3. In September 2017, the results of ACE, the largest pre-diabetes intervention study in China, which lasted nearly 10 years to date, were officially released, confirming that Baitangping can significantly reduce the risk of new-onset diabetes in pre-diabetic population on the basis of lifestyle intervention. (This research is more promotional)
ACE is a multicenter, randomized, placebo-controlled, double-blind, cardiovascular secondary prevention study to assess whether the use of alpha-glucosidase inhibitor acarbose in patients with impaired glucose tolerance in coronary heart disease or acute coronary syndrome can reduce the risk of future CVD.
The study began to recruit participants in October 2008. By October 2015, a total of 6526 patients from mainland China and Hong Kong were enrolled. The participants were older than 50 years old and had a definite diagnosis of coronary heart disease or acute coronary syndrome, which was in accordance with fasting blood glucose < 7 mmol/L and OGTT < 7.8 mmol/L but < 11.1 mmol/L.
Reference: http://news.medlive.cn/endocr/info-progress/show-133055_46.html
4. Acarbose-Glucobay (Acarbose Tablets), the core variety of Bayer in China. It entered the Chinese market in 1994.
Global performance ranged from 350 million euros in 2006-2011. Global performance was 515 million euros in 2016 and 563 million euros in 2017. In 2017, China achieved about 4 billion yuan.
Bayer Pharmaceutical 2018 Q1 reported that sales of diabetes treatment drug Baitangping increased by 13.7% (adjusted by exchange rate) due to the rising market demand in China. Baitangping's performance in 2018 Q2 increased by more than 10% year on year, and Chinese patients contributed a lot.
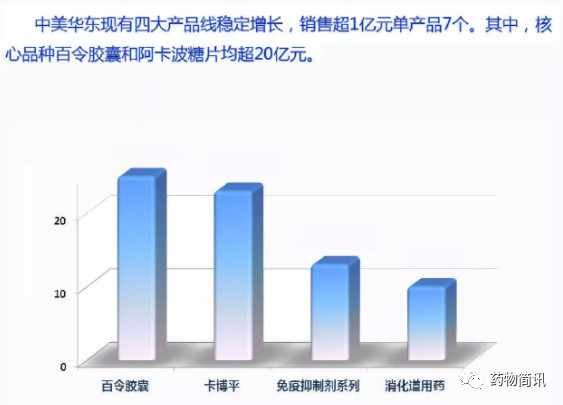
5. Among the domestic generic drugs, East China Medicine, Kabopin (Acarbose):
Income exceeded 1 billion yuan in 2015, sales exceeded 1.5 billion yuan in 2016 and 2 billion yuan in 2017. (annual report caliber)
In addition, on June 2, 2017, Lizhu Group announced that its subsidiary, New Beijiang Pharmaceutical, signed a Material Purchasing Contract with East China on May 31. East China and the United States will purchase Acarbose APIs from New Beijiang Pharmaceutical, with a total price of 480.6 million yuan (including taxes) and a two-year performance period.
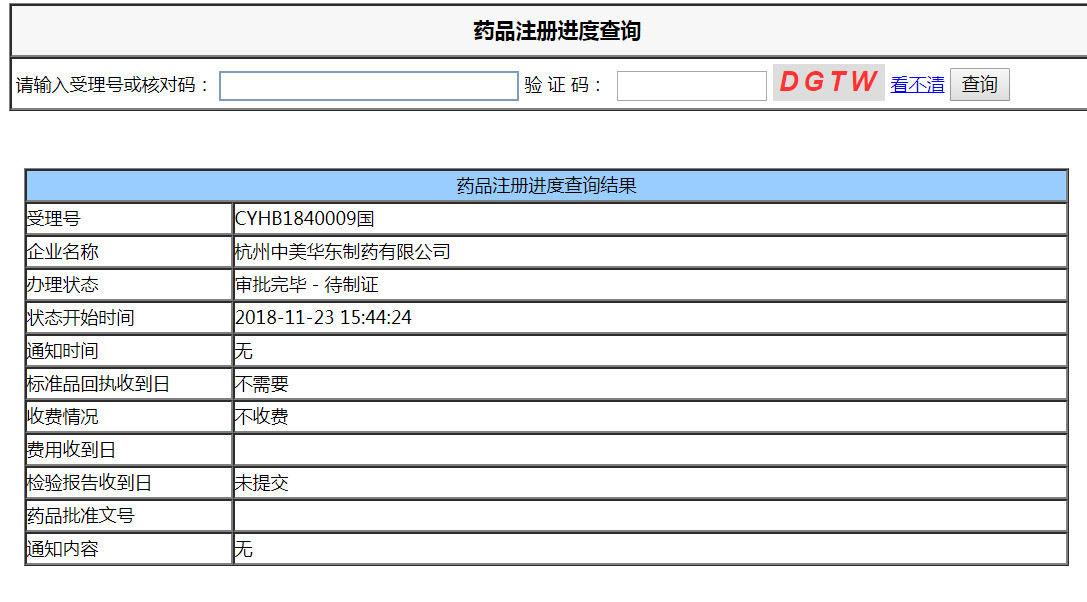
The consistency evaluation of acarbose in East China was completed on April 8, 2018, and submitted to CDE with the acceptance number CYHB1840009. At present, the company BE on-site verification has been completed, waiting for the final review opinion, and is expected to obtain the evaluation results in the fourth quarter of this year.
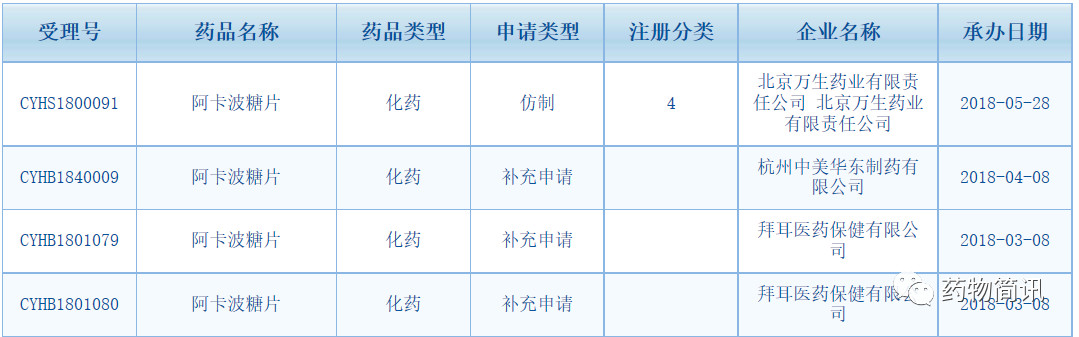
6. Declarations of Acarbose:
In addition to Beijing Wansheng and Taiwan Meishi, companies such as Xintai Pharmaceutical Co., Ltd., Hunan Qianjin Xiangjiang Pharmaceutical Co., Ltd., Hainan Rizhongtian Pharmaceutical Co., Ltd., Sichuan Green Leaf Pharmaceutical Co., Ltd., Haizheng Pharmaceutical Co.
7. Recent data of public hospital terminals in Minet cities show that Bayer accounts for 69% of the market share of acarbose, 26% of Central America and East China, and 5% of Sichuan Green Leaf Baoguang Pharmaceutical Co.
Reference resources:
NMPA/CDE;
Pharnex Data Monitor;
Relevant companies disclose publicly, etc.

