Drug repurposing, commonly referred to in English, refers to the discovery, confirmation and application of new indications for medicines that have been used in clinic (including those that are in use, no longer in use and have been clinically evaluated), which belongs to the category of "old medicines for new use".
The development cycle of innovative drugs is about 10 to 17 years, and the relocation of drug development usually takes only 3 to 12 years. Most of the pre-clinical and human trials of repositioned drugs have proved that they are safe enough, and even the development of preparations has been completed. Uncertainties such as safety and pharmacokinetics were significantly reduced, and development risks were significantly reduced. In addition, the cost of pre-clinical, phase I and phase II trials can be greatly saved.
Successful drug relocation examples include sildenafil, thalidomide, aspirin (analgesia CD / colorectal cancer), zidovudine (cancer HIV), minoxidil (hypertension alopecia), ketoconazole (fungal infection Cushing syndrome), fengomode (transplant rejection MS), rituximab (cancer RA). And so on.
Drug relocation is a feasible strategy for most pharmaceutical enterprises, especially those with small scale but strong R&D capability. In 2011, NIH established the National Center for Translational Science Promotion (NCATS) to fund the reuse of existing drugs and explore possible new uses. The main risks of repositioning drug development lie in clinical effectiveness, patents and markets. Researchers need to return to solid theoretical basis and sufficient experimental data to clarify the mechanism of drug action, and also need to pay attention to patent layout strategy.
Ammonia Lexanol was developed by Takeda Company. It is an anti-inflammatory and anti-allergic immunomodulator that inhibits inflammation by inhibiting the release of histamine and leukotrienes. It was first marketed in Japan in 1987 with eye drops (allergic conjunctivitis) and tablets (allergic rhinitis/bronchial asthma, 20-50 mg, tid/bid). It is approved for use in oral ulcers (5% oral paste) in the United States and has been discontinued for unsafe reasons.
Later, animal studies showed that the drug selectively inhibited TBK1 and IKK-e, resulting in weight loss and improved insulin resistance.
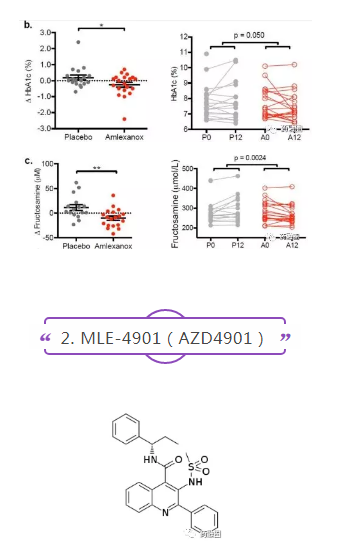
The results of amlexanol in phase II clinical trials (NCT01842282, n = 38) for type 2 diabetes and obesity, a 12-week, randomized, double-blind, placebo-controlled trial, were published in 2017. The main endpoints were the reduction of HbA1c and the improvement of hepatic steatosis. After 12 weeks of treatment (50 mg tid.), HbA1c in the treatment group was 0.5% lower than that in the placebo group on average (P=0.05), and fructosamine levels representing blood sugar control were also significantly lower (P=0.0024). As per bwlow picure:
MLE-4901 is a neurokinin-3 receptor (NK3R) antagonist.
NK3R plays a key role in dopaminergic function and may be involved in the pathogenesis of schizophrenia, which led to the development of several NK3R antagonists such as osanetant and talnetant in the early 20th century. However, many clinical studies failed to prove the effectiveness of NK3R antagonists for schizophrenia, leading to the termination of NK3R drug development.
Postmenopausal women with lower estrogen levels, and hormone deprivation of breast and prostate cancer patients will have hot flashes. Animal studies have shown that neurokinin-3 (NKB) signaling may play an important role in the etiology of hot flashes. In 2015, it was reported that intravenous NKB could induce hot flashes in healthy women.
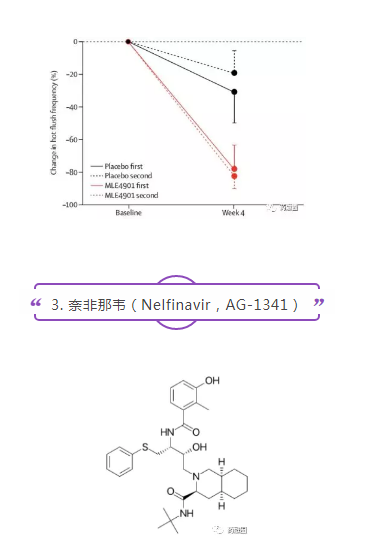
AstraZeneca completed a double-blind, placebo-controlled, cross-design phase II clinical trial of MLE-4901 for hot flashes in menopausal women in 2017 (NCT02668185, n=28). Compared with placebo, oral administration of MLE-4901 reduced the total number of hot flashes per week by 45%. The treatment was well tolerated. Three participants had elevated transaminase activity within 28 days and normalized it within 90 days. The results were published in Lancet magazine.