These Biotech: Get the latest "13th Five-Year Plan" major new drug creation projects!
1,I-MAB BIOPHARMA
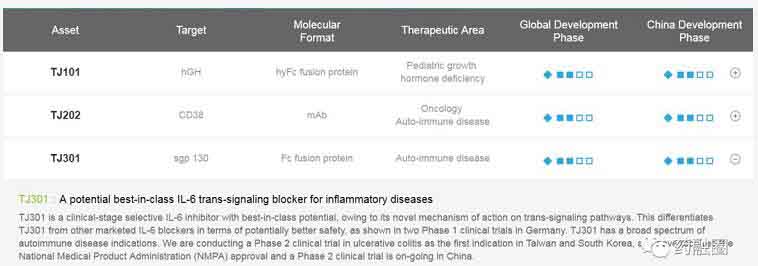
I-Mab Biopharma(Tianjin)Co.,Ltd. The project "International Multicenter Clinical Study of TJ301 in the Treatment of Moderate and Severe Ulcerative Colitis" was established by the National "13th Five-Year Plan" Major New Drug Creation Science and Technology Major Special Project. This project is led by the First Affiliated Hospital of Sun Yat-sen University.
The morbidity of ulcerative colitis (UC) in China is on the rise, and its disability rate seriously affects the quality of life of patients. Traditional medicine can not cure the disease, resulting in repeated attacks of moderate to severe UC. TJ301 is a selective IL-6 trans-signaling inhibitor. Through a new signal transduction mechanism, it does not interact with a single IL-6 or IL-6R. It is expected to be as effective as the existing biological agents acting on the IL-6 pathway, but safer and more suitable for long-term use. It is an anti-inflammatory biological agent for the treatment of ulcerative colitis.
At the end of 2016, Swiss Ferring Pharmaceutical granted Tianjin Biology exclusive rights to develop and commercialize TJ301 (olamkicept) in Greater China and Korea. Conaris Research Institute, the earliest developer of this product, is currently conducting phase II clinical trials of inflammatory bowel disease and ulcerative colitis worldwide.
2, Shanghai Henlius Biotech,Inc.
Shanghai Henlius Biotech,Inc. as the leading unit and responsible unit, was supported by the National Major New Drug Creation Project in the 13th Five-Year Plan by the two projects of "Clinical Research and Industrialization Development of Monoclonal Antibody Large Variety Bioanalogues for Malignant Tumor Therapy" and "Clinical Research and Industrialization Development of Monoclonal Antibody Large Variety Bioanalogues for Autoimmune Disease Therapy".
It is reported that two projects have developed Rituximab, Trastuzul, Bevacizumab and Cetuximab Bioanalogues for malignant tumors such as lymphoma, breast cancer, lung cancer and colorectal cancer, as well as Adamumab and Infliximab for the treatment of autoimmune diseases. Monoclonal antibody and tropizumab biological analogues involve clinical research, optimization and validation of industrialized production process, process control research and quality research in the above fields, and the establishment of national standards.
3,ZAI Lab
ZL-2306 (Nilaparil), an innovative drug urgently needed for the treatment of platinum-sensitive recurrent ovarian cancer, has been approved as a new drug certificate and production license by Zai Lab and received significant special support.
ZL-2306 (Nilaparil) is a highly effective and selective daily oral small molecule poly (ADP-ribose) PARP1/2 inhibitor. Nilaparyl was approved in the United States in March 2017 and in Europe in November of the same year for maintenance treatment of patients with recurrent epithelial ovarian cancer, fallopian tube cancer or primary peritoneal cancer who were completely or partially alleviated by platinum-containing chemotherapy.
Zele (Nilapari) was approved to be listed in Hong Kong in October 2018.
On December 12, 2018, the State Drug Administration (NMPA) formally accepted the application of ZL-2306 (Nilapari) as a new drug (conditionally approved) for the maintenance treatment of adult patients with recurrent epithelial ovarian cancer, fallopian tube cancer or primary peritoneal ovarian cancer who were completely or partially alleviated by platinum-containing chemotherapy.
4,BeiGene
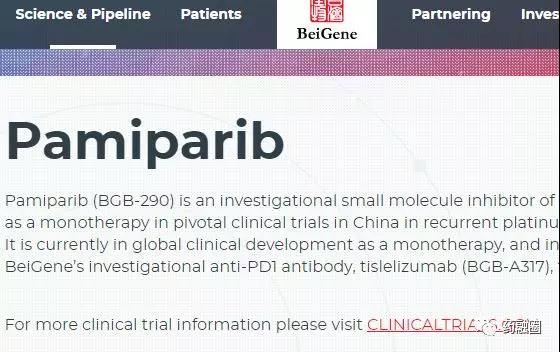
"Clinical Research and Development of PARP Inhibitor BGB-290 for Malignant Tumors" is the third "National Major New Drug Creation Project" supported by Baiji Shenzhou during the 13th Five-Year Plan period.
Pamiparib (BGB-290) is a selective inhibitor of PARP1 and PARP2 independently developed by Baiji Shenzhou. Pre-clinical models show that it has pharmacological properties such as penetrating blood-brain barrier and capturing PARP-DNA complex.
At present, BeiGene is carrying out two key clinical trials of ovarian cancer in China for pamiparib, and three phase clinical trials of gastric cancer in the world. At the same time, BeiGene is also carrying out a phase 1 clinical study of pamiparib combined with tislelizumab in the treatment of solid tumors, phase 1 clinical study combined with temozolomide (TMZ) in the treatment of solid tumors and phase 1 clinical study combined with radiotherapy (RT) and temozolomide in the treatment of glioblastoma.