1. The first NDA applies for oral semaglutide for the treatment of adult patients with type 2 diabetes.
Based on the clinical data of PINEER, including 10 clinical studies, 9543 patients with type 2 diabetes mellitus were enrolled. Controlled trials were conducted with Siglitin (MPP-4 inhibitor of Mosadong), SGLT-2 inhibitor, liraglutide and placebo. The results showed that the blood sugar level of patients treated with oral semaglutide (7 mg and 14 mg) decreased more significantly. Compared with most control drugs, oral semaglutide decreased the average weight more significantly. (The study was published in JAMA).
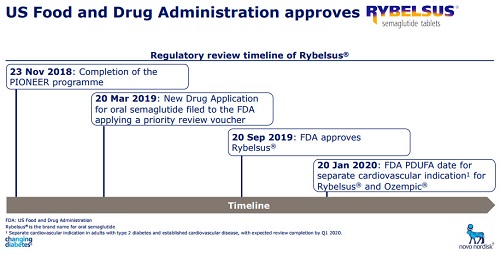
2. In response to the NDA, Novo Nord made a heavy bet, using a Priority Review Voucher (PRV) to speed up the NDA review, which was shortened from 10 months to 6 months. In September 2019, oral somaluronate was approved by the FDA. The name of the product is Rybelsus <, once a day (7mg/14mg) for blood sugar control in patients with type 2 diabetes mellitus.

3. A randomized, double-blind, double-simulated, parallel three-year trial of semaglutide and siglitin once a day was conducted. From February 2016 to March 2018, clinical studies were conducted in 206 regions of 14 countries for 78 weeks. 1864 patients with type 2 diabetes mellitus who used metformin and sulfonylureas to control hypoglycemia were selected from 2463 patients and randomly divided into two groups.
The patients were randomly divided into three groups: oral semaglutide 3 mg group (n = 466), oral semaglutide 7 mg group (n = 466), oral semaglutide 14 mg group (n = 465) and siglitin 100 mg group (n = 467). The initial dose of semaglutide orally was 3 mg/d, up to 7 mg/d every 4 weeks, then up to 14 mg/d until the dose of randomized grouping was reached.
The primary endpoint of this study was the change of HbA1c from baseline to 26 weeks, and the secondary endpoint was the change of body weight from baseline to 26 weeks.
1758 (94.3%) of the 1864 randomized patients (mean age 58, mean baseline HbA1c 8.3%, mean BMI 32.5, 879 women) completed the trial and 298 stopped treatment. HbA1c in 7 mg group and 14 mg group was significantly lower than that in siglitin group (the difference was - 0.3% [95% CI, - 0.4% - 0.1%] and - 0.5% [95% CI, - 0.6% - 0.4%], P < 0.001), and body weight also decreased significantly from baseline to 26 weeks (the difference was - 1.6 kg [95% CI, - 2.0 ~ - 1.1 kg] and - 2.5 kg [95% CI, - 3.0 - 2.0 kg], P < 0.001). Compared with Sigliptin, the two endpoints of somaluptide 14 mg group decreased more significantly at week 78.
At the end of June 2019, Novo Nord's bid for the third phase of clinical trial of oral semaglutide (somalopeptide) versus siglitin (NN9924-4309) in patients with type 2 diabetes mellitus treated with metformin was approved by the genetics office. Peking University People's Hospital. The excellent clinical data of oral semaglutide are expected to end soon in China.

