5,Antengene Corporation
Antengene Corporation 's "Clinical II/III Phase I Development of ATG-008, a Class I New Drug for Hepatocellular Carcinoma Acting on TORC1/2 Dual Targets", received special support from the State for major new drug creation during the 13th Five-Year Plan period, and also received the first central financial support fund for 2018. Selected "Major New Drug Creation Project" as the world's first new drug for hepatitis B virus-positive advanced hepatocellular carcinoma.
The study showed that the median survival time of ATG-008 was 12.1 months for patients with hepatitis B virus-positive hepatocellular carcinoma who were treated with sorafenib and failed chemotherapy, while that of patients with hepatitis B virus-negative hepatocellular carcinoma was 5.2 months. ATG-008 has also obtained the approval of clinical trials including Taiwan, China and Korea, and started to administer drugs to patients in group in August this year. All clinical studies are being carried out smoothly.
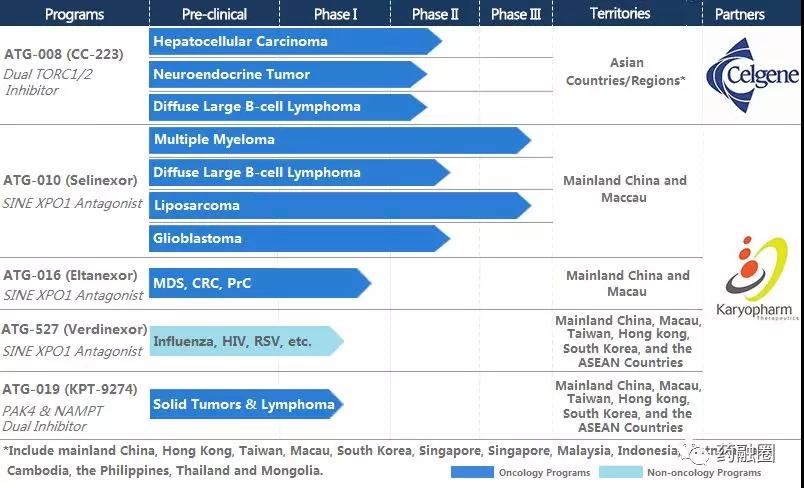
On January 2, 2019, the company just disclosed that it had completed a $120 million B round of financing.
6,YISHENG BIOPHARMA
The world's first pika rabies vaccine based on biology research and development was selected as the national "13th Five-Year Plan" major new drug creation technology major special project. This project was supported again by the national major new drug creation project in 2013. This project will support the large-scale overseas three-phase clinical trials of the product, the project name is "lyophilized human pika rabies vaccine (LYD-PRV)". Phase III clinical trial of Vero cells in Southeast Asia. At present, the product has completed Phase I and Phase II clinical trials overseas, and is about to enter Phase III clinical development stage.

Rabies is an infectious disease with a fatality rate of nearly 100%. Every year, about 60,000 people worldwide die from rabies, covering 100 countries and regions. As a new generation of rabies vaccine, Pika rabies vaccine uses a new immune regulation activation technology, through activating Toll-like receptor 3 (TLR-3) and other pathways, quickly induces strong cellular and humoral immunity, protects the body from rabies virus infection, and greatly improves the protection rate of the vaccine. Compared with the current standard immunization protocol for rabies vaccine, which requires multiple injections in four weeks, the immunization protocol for three injections a week will greatly improve the compliance of the immunized population. The products and related technologies have been patented in more than 60 countries, covering the United States, China, the European Union and other major countries.
7;Zensun Sci & Tech Co., Ltd
Zensun Sci& Tech Co.,Ltd.The Heart Failure Project, The Multinational Multicenter Phase III Clinical Study of Recombinant Newland Green, an Innovative Anti-Heart Failure Drug
01-010-004) was supported by the National Major New Drug Creation Project in the 13th Five-Year Plan, and received the first central financial support fund in 2018.
Recombinant anthuman Neuregulin-1 (rhNRG-1, Neucardin #1) for injection is the first international innovative drug and a kind of biological product independently developed by Shanghai Zesheng Science and Technology Development Co., Ltd. It is expressed by microorganisms, separated and purified, and then lyophilized for the treatment of chronic heart failure. By binding with receptors on the surface of myocardial cells (ERBB2/ERBB4), it activates downstream cMLCK (myosin-specific light chain kinase), phosphorylates MLC-2V (myosin light chain), reshapes the structure of myocardial cells, and regulates the calcium balance in myocardial cells, thereby improving the contraction/diastolic function of myocardium, reversing the process of cardiac remodeling, and reducing mortality. It is essential to treat chronic heart failure.
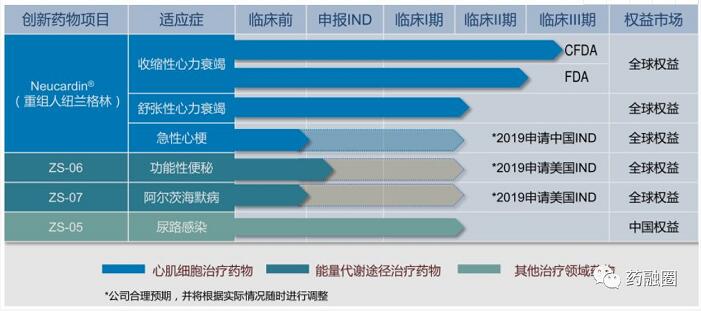
The major project of the 13th Five-Year Plan aims to continue to complete phase III clinical research of the United States/International Multicenters on the basis of previous work to confirm the validity and safety of Newland Green, and to submit applications for new drug listing of biological products (BLA) to drug regulatory agencies in international markets such as FDA of the United States. (New drugs with a long time span)
8,IMPACT THERAPEUTICS
IMPACT THERAPEUTICS 's "Clinical Research on the New Targeted Anticancer Drug PARP Inhibitor IMP4297" was supported by the "Major New Drug Creation Project of 135 Countries".
British Pharmaceutical successfully completed a $30 million C round of financing last August. At a rough glance, three PARPs alone were selected...
9,ACEA Pharma
ACEA Pharma. undertook the project "Registered clinical research and large-scale production process development of the first original third-generation EGFR targeting anti-lung cancer new drug ivetinib maleate", which was established by the National "13th Five-Year Plan" major new drug creation science and technology major special projects.
In August 2018, Ivetinib's application for new drug marketing has been included in the priority review procedure of the State Drug Administration, and the production base meeting the requirements of GMP has been built.

